Calcium signalling and cardiovascular pathophysiology
GOMEZ Ana Maria, DR1 Inserm : Team leader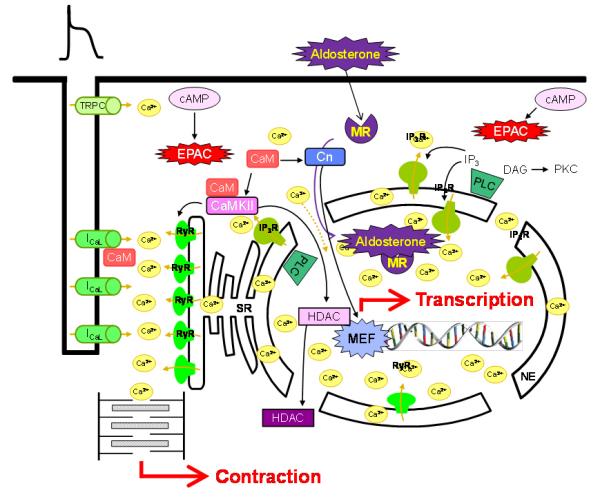
BENITAH Jean-Pierre, DR2 Inserm : Team leader
MERCADIER Jean-Jacques, PU-PH Emérite
MOREL Eric, PU Université-Paris-Saclay
SABOURIN Jessica, MCU Université-Paris-Saclay
PERRIER Romain, MCU Université-Paris-Saclay
PEREIRA Laetitia, MCU Université-Paris-Saclay
POILBOUT Corinne, IE Inserm
GERARD Garance, PhD Student
LE GOURRIEREC Pauline, PhD Student
LUO Shuai, PhD Student
OUGHLIS Célia, PhD Student
TEBOULL Sarah, PhD Student
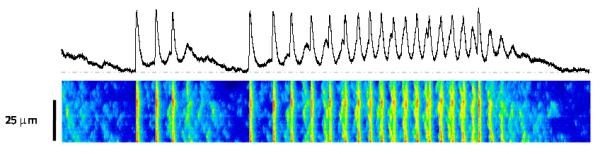
Cardiovascular diseases remain the leading cause of death in developed countries. Last stage of cardiac pathologies, heart failure (HF) is major cause of morbidity and mortality. Despite therapeutic improvement, HF patient's prognostic is very poor, and more than 50% die suddenly as consequence of ventricular arrhythmia. This fact is the consequence of misunderstanding of the molecular mechanisms responsible of contractile dysfunction and arrhythmogenesis. In this sense, in addition of activating cardiac contraction, Ca2+ is recently emerging as a key factor in transcription regulation (excitation-transcription coupling, ETC) and in arrhythmia development. In order to better understand HF syndrome, we develop a project program focused in analyzing Ca2+ handling involvement in the genesis of HF and arrhythmia. Our project aims to elucidate the adaptive and maladaptive mechanisms involved in cardiac hypertrophy and arrhythmia. It is focused in:
1/ small G proteins and their exchange factors with a special focus on a cAMP-directly activated protein named Epac, whose expression is increased in HF. We have shown Epac involvement in calcium homeostasis. Our aim is to elucidate its spatio-temporal mechanisms involved in hypertrophy and in the ETC.
2/ Aldosterone, which is involved in arrhythmia independently of its pressor effects. We have shown a major role of this hormone in Ca2+ handling. We will analyze its role in HF and arrhythmia.
3/ Mutations in the Ca2+ release channel (RyR) responsible of cathecolaminergic polymorphic ventricular tachycardia (CPVT). We have shown functional consequences of a mutation in the C terminal portion of the RyR. Our project is to analyze a new mutation in the N-terminal portion of the channel. These analyses will not be limited to the ventricle, but will be extended to the sinus tissue.
A new axis consists in analyzing the mechanisms involved in cardiotoxicity of anti cancer therapy. Indeed, recent epidemiologic data have shown that patients treated for cancer therapy present delayed radio or chemo-induced cardiomyopathies with a high mortality rate. The current evolutions of treatment's standard toward combined therapies might exacerbate the toxic effects. Thus, cardiotoxicity would become a major concern within the next years. This translational project will be performed in collaboration with the Gustave Roussi Institute (GRI) for the clinical part (INSERM Unit 1030) and our team for bench side. The aims of this partnership are to 1/ elucidate the radio and chemo-induced physiopathological and molecular basis of these cardiomyopathies with a special interest for the small G proteins and Epac 2/ identify the predictive molecular biomarkers leading to the recognition of patients susceptible to develop delayed side effect in the cardiac physiopathologic field.
Technics :
Electrophysiology : patch-clamp and lipid bilayers
Confocal Microscopy (alone or coupled to patch-clamp)
Biochemistry and molecular biology.
Transgenic animal breeding
Keywords : heart, excitation-contraction coupling, RyR, Ca2+, sparks, Ca2+ current, Epac, small G proteins, aldosterone, arrhythmia, heart failure.







 Subscribe to RSS Feed
Subscribe to RSS Feed